The pharmaceutical industry has been abusing the patent system for decades. One of the reasons why their practices have gone unchecked for so long is because regulators, policymakers, competitors, and the public do not have easy access to data that provide a comprehensive picture of how pharmaceutical patenting actually works in practice and how it impacts drug prices.
The Orange Book—a database of important information on FDA-approved drugs that includes a selection of relevant patents—is one of the few tools stakeholders have to track pharmaceutical patenting activity. However, it only lists a sliver of patenting information. The Orange Book lists *some patents* on FDA-approved drugs, but not every patent that may pose an obstacle for competitors when developing their generic versions, or which could be asserted against them in litigation. As one Chief Counsel for the FDA has said, “the Orange Book should not be relied upon to identify the complete range of patent claims that can be asserted”. Biologic drug patents are listed on the Purple Book, which has its own limitations when it comes to transparency.
| READ: Investigating the Pharmaceutical Industry’s Drug Patenting Practices |
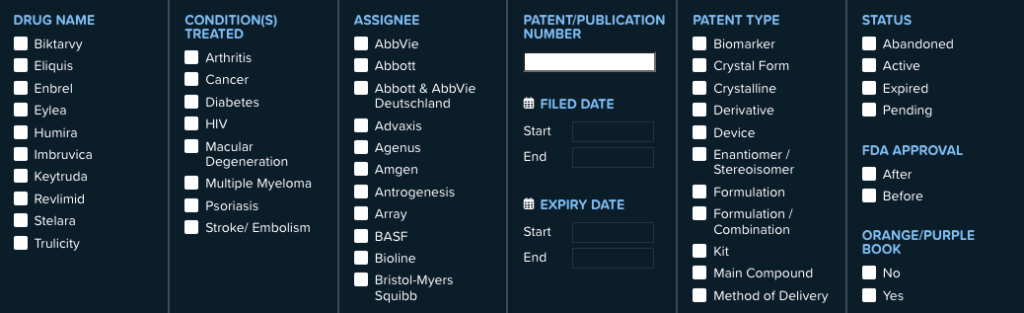
Regulators, policymakers, competitors, and the public need to be able to understand the full extent of the patenting tactics of branded drug companies. The Orange Book only provides a limited view, making any policy recommendations or reports based solely on it incomplete and potentially misleading. To provide a more comprehensive picture, our team of scientists, practitioners, patent informatics and analytics experts, and IP attorneys analyzed patents on some of the top-selling drugs in the U.S. and published our findings and methodology in a publicly-accessible database, The Drug Patent Book.
We pored over thousands of pages of published patent documents to determine which patents might protect a drug and how it is manufactured, as well as regulatory documents, and court records. Just because patents are published and available publicly does not mean there is transparency. They still have to be analyzed to see what each one covers. What we uncovered was shocking: thousands of filed and granted patents on top-selling drugs like Keytruda, Enbrel, Humira, and Revlimid, many of which are hidden from regulators and policymakers. We discovered 1,429 filed and granted patents on the 10 top-selling drugs in the U.S. in 2021. Our research is ongoing and we will be updating our database as well as publishing new reports about patent abuse this year.
Patent abuse thrives in the shadows. At I-MAK, our mission is to bring it into the light.
In solidarity,
Tahir
What We’re Reading and Watching:
- Last month, we explained how Merck will attempt a product hop by shifting 30-40% of all Keytruda patients to a patent-protected subcutaneous version. Ironically, the Wall Street Journal recently reported that the subcutaneous version of Keytruda may infringe on technology claimed in patents held by biotech company Halozyme.
- In our new video, we tell you how the consulting firm McKinsey helped the pharmaceutical company AbbVie abuse the patent system to turn Humira into one of the most lucrative drugs in history. This egregious overpatenting is not just an AbbVie and McKinsey issue—it’s industry standard.
- On The Daily Show, Jon Stewart explained how Americans are getting ripped off by the pharmaceutical industry: “Pharmaceutical companies get everything from our government—tax breaks, research grants, patent extensions—worth billions of dollars. And what do we the people get for it? The highest drug prices in the Western Hemisphere.”